By Dr. Anthony C. Pearson, MD, FACC
The 12-lead ECG was the first non-invasive tool available to cardiologists to make the premortem diagnosis of left ventricular hypertrophy (LVH). ECG diagnosis of hypertrophy is frequently reported and remains an important part of evaluating cardiac patients to this day.
Most of the dozens of ECG criteria developed in the last fifty years for diagnosing LVH have relied on the magnitude of the voltage in limb and precordial leads. The Cornell and Sokolow-Lyon criteria, which exclusively utilize ECG voltage, track echocardiographic left ventricular mass better, but their sensitivity is still unacceptably low—to the point where some authors have declared the assessment of ECG LVH clinically useless.
However, recent studies suggest that information contained within the standard ECG beyond voltage can provide important insights into myocardial structure and function.
Incorporating ECG Information Beyond Voltage
Back in 1968, Romhilt and Estes published a different approach to utilizing ECG findings to diagnose hypertrophy.1 Their system, called the R-E score, incorporated multiple ECG components, including ST changes, P wave changes, and QRS axis. ECGs with five points from this six-point scoring system are considered to have LVH.
As Estes and others pointed out in the American Heart Journal in 2015, the sensitivity was low, and "attempts to improve the sensitivity of R-E score proved fruitless, as each such modification led to an unacceptable increase in false positives. The advent of and widely increased availability of imaging technology has made optimizing current ECG LVH criteria less relevant."2
Work on refining ECG criteria for LVH has continued, with researchers identifying additional parameters that may be helpful. A study published in 2022 Scientific Reports, showed the spatial QRS-T angle had better sensitivity than conventional criteria for identifying moderate or greater LVH in patients with at least moderate hypertrophy on imaging and was associated with adverse cardiovascular outcomes in patients with underlying cardiac comorbidities.3
If conventional LVH criteria are decreasingly relevant, what should cardiologists be paying attention to in the ECG?
Defining ECG Strain
An important component of the R-E score is ST strain, which Estes defined as "ST segment and T wave in opposite direction to QRS in V5 or V6, without digitalis." It garnered three points, equivalent in value to the R-E voltage component (R or S wave in any limb lead ≥2 mV, or S wave in V1 or V2 ≥3 mV, or R wave in V5 or V6 ≥3 mV).
ECG strain has also been more recently defined in a 2017 paper in the Journal of the American Heart Association (JAHA) as "coexistence, in leads I, II, aVL, or V3 to V6 of ST-segment horizontal or downward sloping depression ≥0.05 mV plus negative T wave" (Minnesota code 4–1 or 4–2 and 5–1 or 5–2).4
The precise cause of underlying ECG strain is unknown. It has been hypothesized in a 2014 Circulation study that it is a marker of "LV decompensation," and recent studies confirm a high association with markers of LV fibrosis or scarring.5
More recently, researchers writing in 2021 in JAHA said that an LV electrocardiographic strain pattern "is presumably the electrocardiographic translation of chronic myocardial oxygen imbalance" stemming from changes to coronary perfusion from increased LV mass and "adverse LV geometric change, function, and fibrosis in response to aortic stenosis." 6
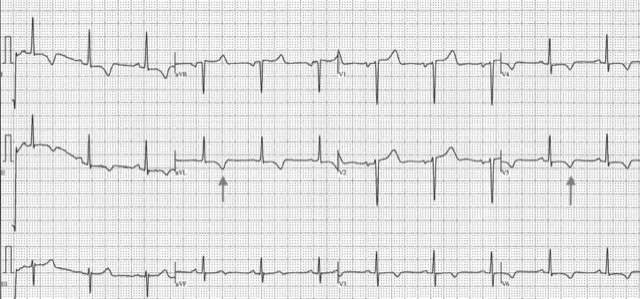
This ECG* demonstrates strain pattern in leads I, aVL, V5, and V6. The patient had severe concentric LVH by echo, but no ECG voltage criteria for LVH.
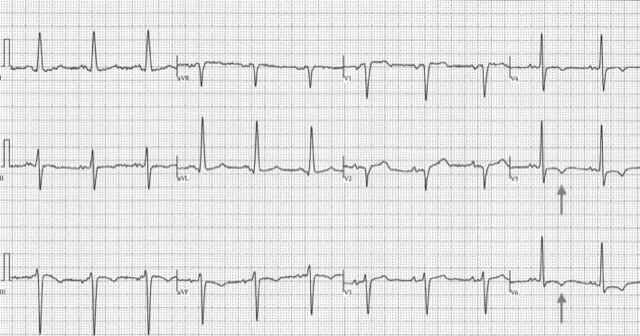
This ECG* demonstrates a strain pattern isolated to V5 and V6. In addition, classic voltage criteria for LVH are present—Cornell criteria >28 mm in RaVL (19 mm) and S V3 12 mm—along with left axis deviation and left atrial enlargement, fulfilling Romhilt-Estes criteria for ECG LVH. The patient had moderate concentric LVH by echo, normal coronaries, and recent decompensated heart failure.
To learn more about the power of the ECG in today's clinical landscape, browse our Diagnostic ECG Clinical Insights Center.
ECG Strain and Prognosis
Multiple studies have demonstrated that ECG strain is an important independent prognostic finding beyond its contribution to diagnosing LVH.
A 2008 review in the Cardiovascular Journal of Africa showed that the ECG strain pattern was associated with heightened risks of all-cause and CV morbidity and mortality, with similar relationships observed in studies conducted since then.7
A study in the American Journal of Cardiology published in 2017 showed that ECG strain—found in 28% of patients undergoing surgical aortic valve replacement for aortic stenosis (AS)—was associated with worse 8-year survival, regardless of the presence of LVH.8
In 2018, researchers reported in Structural Heart that the ECG strain pattern in patients with LVH and calcific aortic valve disease was associated with a greater risk of all-cause mortality.9
Similarly, in the 2021 JAHA study—which included patients undergoing transcatheter aortic valve replacement—there was a link between ECG strain pattern and a 2.75-fold greater risk of rehospitalization for heart failure in those with or without LVH. There was no relationship with overall or cardiac death, however.
ECG Strain in Aortic Stenosis
Recent studies utilizing cardiac MRI have examined ECG strain in relation to myocardial tissue changes in patients with AS. As these patients transition from hypertrophy to heart failure, there is progressive myocyte cell death and myocardial fibrosis. Cardiovascular magnetic resonance (CMR) can quantify and visualize this mid-wall fibrosis, which has been associated with LV decompensation and adverse prognosis in patients with AS.
The 2014 Circulation study followed 140 Scottish patients with AS for 10.6 years. Compared to patients without ECG strain, patients with ECG strain had more severe AS, higher LV mass index, high troponin I levels, and more diffuse fibrosis by CMR.
All patients with ECG strain had mid-wall late gadolinium enhancement, which was independently associated with ECG strain. ECG strain was an independent predictor of aortic valve replacement or cardiovascular death.
These same investigators went on to develop and validate a novel clinical score using variables associated with mid-wall myocardial fibrosis on CMR, which was published in 2016 in the European Heart Journal.10 The primary outcome predicted by the score was aortic-stenosis-related events (CV death, heart failure, angina, dyspnea, or syncope).
The factors that were found to be independent predictors in patients with AS were age, sex, maximum aortic velocity from Doppler, high-sensitivity troponin I concentration, and ECG strain (presence of ≥1 mm concave down sloping ST depression with asymmetrical T-wave inversion in the lateral leads).
AS related events were more than tenfold higher in patients with a high score versus those with a low score.
ECG Strain in Apparently Healthy Individuals
The 2017 JAHA study examined the significance of ECG strain in a cohort of 6,441 participants without cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis trial.
ECG strain was found to be an independent predictor of all‐cause death, incident heart failure, myocardial infarction, and incident cardiovascular disease (CVD) over 10 years in multiethnic participants without past CVD. In addition, ECG strain was associated with the development of LV concentric remodeling, a decline in LV systolic function, and LV myocardial scar (demonstrated on CMR) after 10 years of followup, independent of voltage criteria for LVH.
The authors concluded that ECG strain is an "early marker of LV structural remodeling that contributes to the development of adverse cardiovascular events." This study extends the prognostic value of ECG strain in predicting adverse cardiovascular events to apparently healthy individuals in multiethnic populations.
ECG strain provides powerful insights into the status of the LV myocardium in populations at risk of LV decompensation: it appears to be a specific marker of mid-wall left ventricular myocardial fibrosis and predictive of adverse clinical outcomes in seemingly normal patients and patients with AS before and after AVR.
Since strain identifies early myocardial decompensation, clinicians should consider treating individuals with ECG strain aggressively for modifiable risk factors of LVH, regardless of the presence of ECG voltage criteria for LVH.
*Anonymized images provided by and published with permission from Dr. Anthony Pearson.
Resources:
1. Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. American Heart Journal. June 1968; 75(6): 752-758. https://www.sciencedirect.com/science/article/abs/pii/0002870368900355
2. Estes EH, Zhang ZM, Li Y, et al. The Romhilt-Estes left ventricular hypertrophy score and its components predict all-cause mortality in the general population. American Heart Journal. April 2015; 170(1): 104-109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4646417/
3. Maanja M, Schlegel TT, Kozor R, et al. Improved evaluation of left ventricular hypertrophy using the spatial QRS-T angle by electrocardiography. Scientific Reports. September 2022; 12(1): 15106. https://www.nature.com/articles/s41598-022-16712-3
4. Inoue YY, Soliman EZ, Yoneyuma K, et al. Electrocardiographic strain pattern is associated with left ventricular concentric remodeling, scar, and mortality over 10 years: the Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. September 2017; 6(9): e006624. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5634304/
5. Shah ASV, Chin CWL, Vassilou V, et al. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. August 2014; 130: 1607-1616. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.114.011085
6. Heger J, Trimaille A, Kibler M, et al. Electrocardiographic strain pattern is a major determinant of rehospitalization for heart failure after transcatheter aortic valve replacement. Journal of the American Heart Association. February 2021; 10(3): e014481. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7955442/
7. Ogah OS, Oladapo OO, Adebiyi AA, et al. Electrocardiographic left ventricular hypertrophy with strain pattern: prevalence, mechanisms and prognostic implications. Cardiovascular Journal of Africa. January-February 2008; 19(1): 39-45. https://journals.co.za/doi/10.10520/EJC23083
8. Guinot B, Magne J, Le Guyader A, et al. Usefulness of electrocardiographic strain to predict survival after surgical aortic valve replacement for aortic stenosis. American Journal of Cardiology. July 2017; 120(8): 1359-1365. https://www.ajconline.org/article/S0002-9149(17)31186-4/fulltext
9. Prihadi EA, Leung M, Vollema EM, et al. Electrocardiographic pattern of left ventricular hypertrophy with strain and survival in calcific aortic valve disease. Structural Heart. May 2018; 2(3): 240-246. https://www.structuralheartjournal.org/article/S2474-8706(22)00158-0/fulltext
10. Chin CWL, Messika-Zeitoun D, Shah ASV, et al. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. European Heart Journal. February 2016; 37(8): 713-723. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4761400/
Dr. Anthony C. Pearson, MD, FACC is a Professor of Medicine at the St. Louis University School of Medicine Division of Cardiology and specializes in general and noninvasive cardiology.
The opinions, beliefs and viewpoints expressed in this article are solely those of the author and do not necessarily reflect the opinions, beliefs and viewpoints of GE Healthcare. The author is a paid consultant for GE Healthcare and was compensated for creation of this article.