Survival rates of various cancers have been improving in recent decades due to better therapeutics. However, this also means treatment-related side effects, like cardiovascular complications and toxicity, take on even greater importance.
A new specialty, cardio-oncology, has developed in recent years to meet the cardiovascular needs of this growing population of cancer survivors in terms of both treatment side effects and preexisting heart conditions. There are a variety of diagnostic tools at physicians' disposal to help diagnose cardiovascular disease in patients with cancer, with the ECG maintaining a vital role in this setting.
Defining Cardiac Toxicity and How ECG Helps
With patients living longer after a cancer diagnosis, cardio-oncologists need to focus more on the effects of treatment on the heart. The International Cardio-Oncology Society (IC-OS), citing a lack of standardization in definitions for cardiovascular toxicity, released a consensus statement to provide some clarity.1 The document covers five key side effects of cancer therapies: cardiac dysfunction/heart failure, myocarditis, vascular toxicity, hypertension, and arrhythmias/QTc prolongation.
The ECG is a vital tool involved in the diagnosis of myocarditis, which has been identified as a complication of treatment with immune checkpoint inhibitors, doxorubicin, fluorouracil, cyclophosphamide, and radiation. But ECG is perhaps most useful when it comes to identifying heart rhythm problems associated with cancer therapy, including supraventricular and ventricular arrhythmias and accurately measuring QTc prolongation.
"The full scope of abnormalities in cardiac electrophysiology can be seen in patients with cancer. These may be related to cancer therapy, underlying predisposition/risk, or both," the authors of the consensus statement note. They add that Afib is commonly seen with cancer treatment, but how Afib and other arrhythmias are defined within this population is the same as it is in the broader population.
Assessing QTc Prolongation
The IC-OS document provides a standardized definition of QTc prolongation in the presence of various cancer therapies since existing studies have classified it in a number of ways. Several medications used to treat cancer have been associated with QTc prolongation, including arsenic trioxide, HDAC inhibitors, tyrosine kinase inhibitors, and cyclin-dependent kinase 4-6 inhibitors, for example.
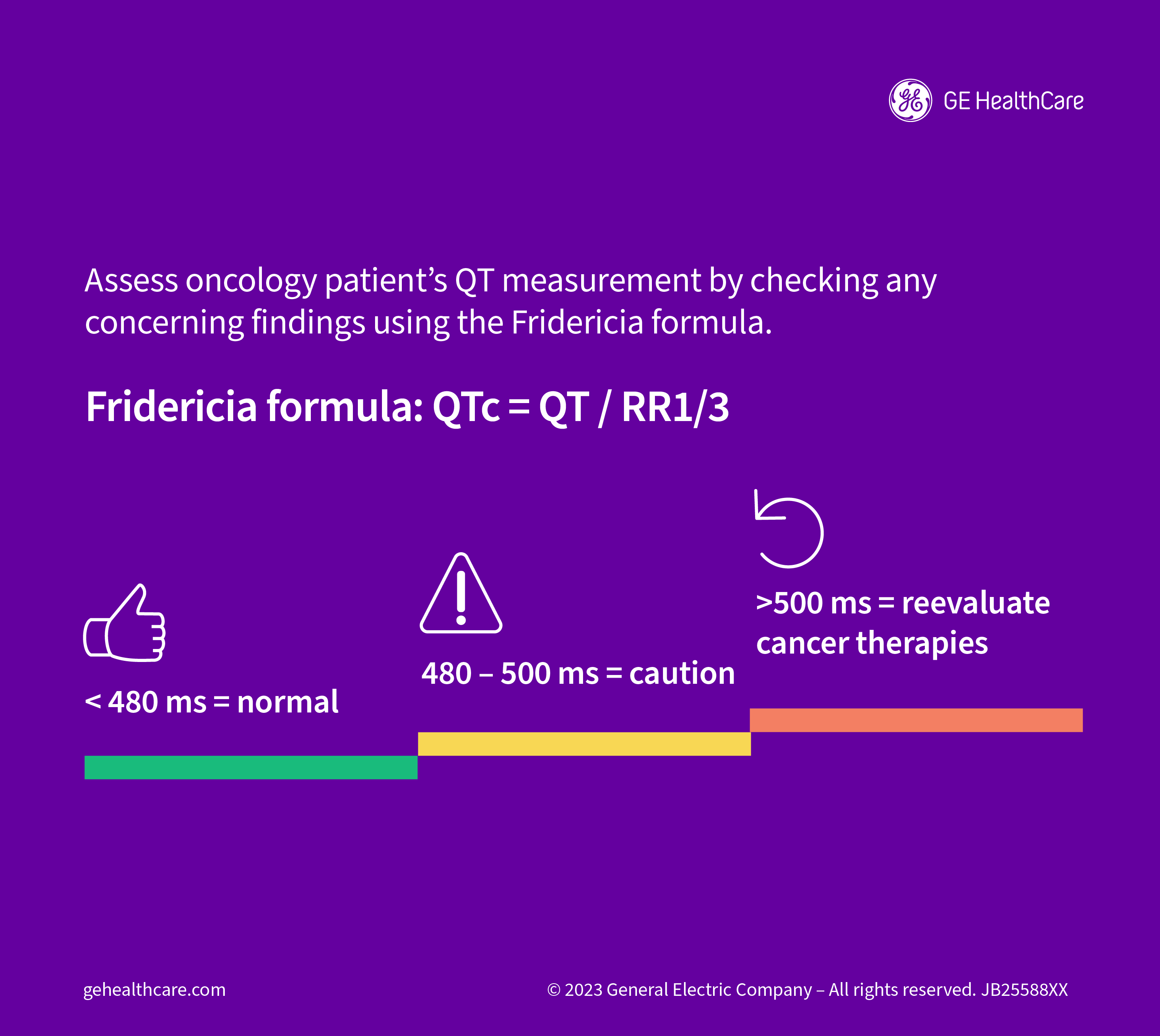
The IC-OS recommends using the Fridericia formula to calculate QTc interval due to its relative ease of use, with prolongation defined as a QTc interval greater than 500 ms. This definition "is based on epidemiologic data demonstrating increased risk of arrhythmias and can be applied universally to all cancer therapies, which will significantly improve and simplify care delivery."
Automated QT measurements are acceptable in most circumstances, as long as the ECG machines have been programmed to use the Fridericia formula. That said, the authors advise that a physician with expertise in treating cardiac issues in patients with cancer should manually check any concerning findings. "This is particularly true for patients with ventricular pacing or bundle branch blocks, as the associated QRS prolongation must be accounted for when assessing the QT interval."
The IC-OS document also provides a recommended approach to applying the measured QTc interval to clinical decisions around cancer therapies. If the value is less than 480 ms, it's considered normal, whereas a value between 480 and 500 ms warrants caution. When the QTc interval exceeds 500 ms, the physician should reevaluate the treatment approach.
"It is important to remember that the QT interval is not stagnant and should be reassessed if the clinical status (e.g., electrolyte disturbances) of the patient changes or dose changes have been applied," the authors say.
Pre-Treatment Assessments with ECG
The ECG is part of a baseline evaluation in patients with cancer, along with patient history, physical exam, testing of cardiac biomarkers, and echocardiography, according to a study in Cancers, which discusses whether ECG may be helpful in identifying patients with cancer who will go on to develop cardiovascular toxicities before they start treatment.2
"ECG analysis is a recommended component of each cardio-oncological evaluation because malignant arrhythmias are frequent side effects of many cancer therapies," the researchers write. "Early identification of ECG abnormalities associated with structural or electrical alterations is therefore of great importance."
They evaluate whether an ECG score that was originally developed to predict sudden arrhythmic death in patients with CAD could also predict cardiotoxicity in treatment-naïve patients with cancer. The score encompasses four ECG parameters—contiguous Q waves, markers of left ventricular hypertrophy, QRS duration, and JTc prolongation—and ranged from zero to five points.
Among 134 patients who received treatment at two clinics in Germany, the incidence of cardiotoxicity over a year of follow-up after treatment initiation was 21% overall, increasing from no cases of cardiac harm in patients with a score of zero to a rate of 7.5% in those with a score of one, 55% in those with a score of two, and 71% in those with a score of three. The only patient with a score of four developed toxicity, while the only patients with a score of five did not. The rate of cardiotoxicity was 57.1% among those with a score of at least two, and 5.4% in patients with lower scores.
"Scoring could help to identify at-risk patients independently or with respect to their planned chemotherapy regimen and to individualize follow-ups and cardioprotective therapies," the researchers say. "Risk assessment by scoring systems is a part of personalized precision medicine and therefore should be part of modern cardio-oncology."
What the Guidelines Say
Several professional societies have released guidance touching on the identification, monitoring, and treatment of cardiovascular conditions in patients with cancer, although the European Society of Cardiology (ESC), in collaboration with the IC-OS and other groups, just released the first comprehensive guideline specifically focused on cardio-oncology in 2022.3 Prior to that, the ESC had published a position paper on cardiovascular toxicity stemming from cancer treatments.4
The ECG plays an integral role in various aspects of care in this setting, according to the new guideline. There is a class I recommendation for all patients with cancer to receive a baseline cardiovascular toxicity risk assessment, which involves at a minimum a clinical assessment and ECG with other tests reserved for select patients based on baseline toxicity risk and specific treatment type.
When the baseline ECG reveals an abnormality, such as advanced conduction disease, Q waves in at least two contiguous leads, left ventricular hypertrophy, previously undiagnosed Afib/atrial flutter, or QTc prolongation, the authors advise that patients be referred to a cardiologist.
There is also an ongoing need for ECG, according to the ESC. It makes another class I recommendation for an annual cardiovascular risk assessment that includes a clinical review, ECG, and measurement of blood pressure, lipids, hemoglobin A1C (HbA1C), and natriuretic peptides for cancer survivors "who were treated with a potentially cardiotoxic cancer drug or radiation therapy to a volume exposing the heart," regardless of the estimated risk level.
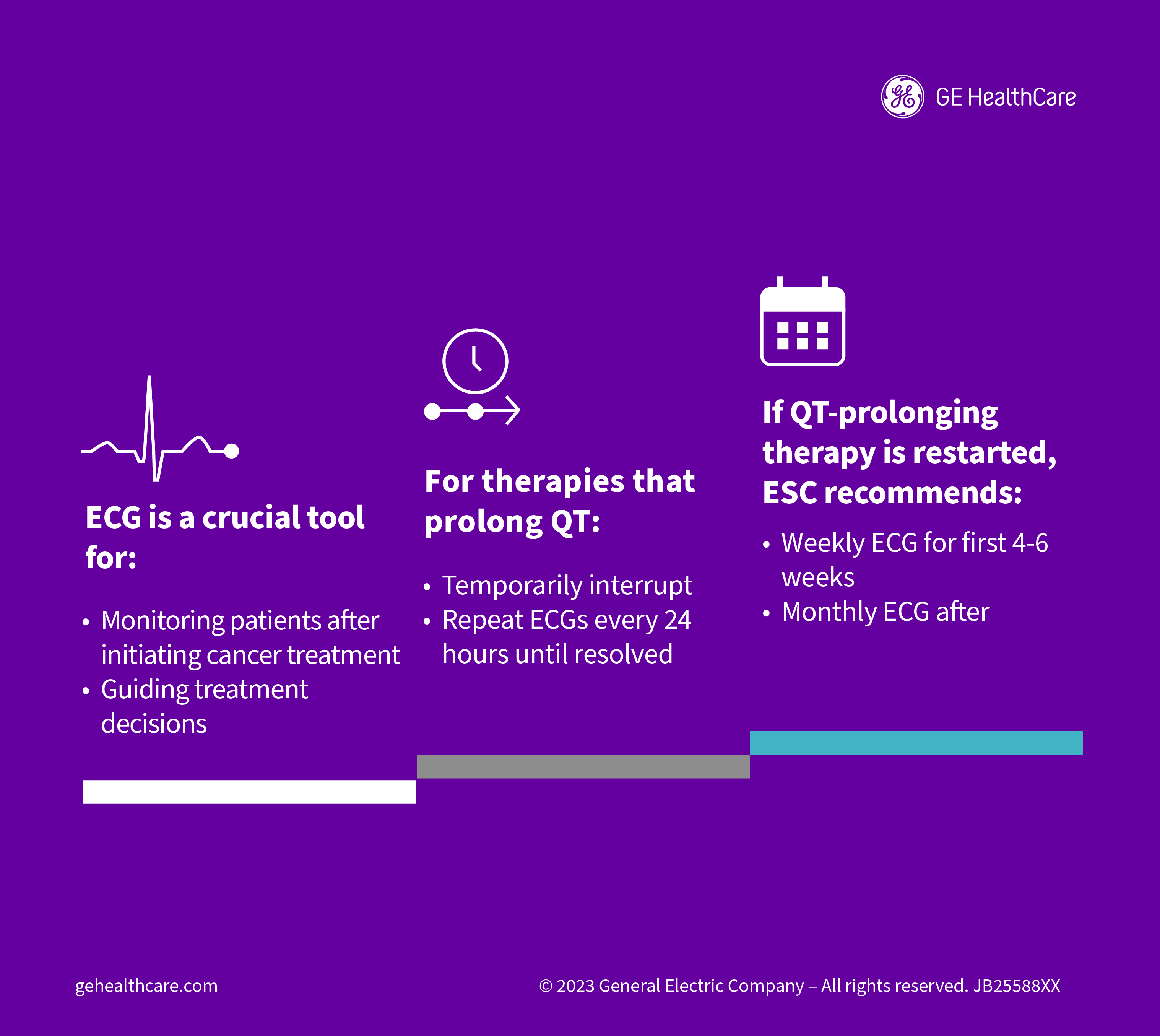
Peppered throughout the document, the ECG is highlighted as one of the crucial tools for monitoring how patients are doing after initiating treatment for cancer and guiding treatment decisions. The authors recommend, for instance, therapies that prolong the QTc should be temporarily interrupted, with repeat ECGs performed every 24 hours until the issue is resolved. If QTc-prolonging therapy is then restarted, the ESC recommends weekly ECG monitoring for the first four to six weeks followed by monthly ECGs.
The ECG and Cancer Prognosis
Measuring the ECG can also give cardio-oncologists an idea of how their patients with cancer will fare. A study in the European Journal of Heart Failure (EJHF) showed that an elevated resting heart rate, measured with a 12-lead ECG after 10 minutes of supine rest, was associated with worse survival in patients with colorectal, pancreatic, or non-small cell lung cancer—and most had previously received chemotherapy.5
To eliminate treatment's potential impact on survival, the same research group later performed a similar study in treatment-naïve patients with a variety of cancers. According to results also published in the EJHF, there remained a strong relationship between resting heart rate and all-cause mortality, particularly in patients with lung and gastrointestinal (GI) cancers.6
The ECG can provide important prognostic information to survivors of childhood cancer as well. A study in the American Heart Journal showed that at a median age of 31, survivors were more likely than their peers from the community to have major ECG abnormalities, including ST/T wave abnormalities, evidence of acute myocardial infarction (MI), and left ventricular hypertrophy with strain pattern.7 The presence of such findings was associated with a fourfold greater risk of all-cause mortality.
Overall, the ECG has a prominent role in cardio-oncology, spanning initial risk assessments, therapeutic monitoring, and prognosis predictions for patients with cancer who receive potentially cardiotoxic treatments.
Resources
- Herrmann J, Lenihan D, Armenian S, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. European Heart Journal. January 2022;43(4):280-299. https://academic.oup.com/eurheartj/article/43/4/280/6460956.
- Pohl J, Mincu RI, Mrotzek SM, et al. ECG scoring for the evaluation of therapy-naïve cancer patients to predict cardiotoxicity. Cancers. March 2021;13(6):1197. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7999575/.
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). European Heart Journal. November 2022;43(41):4229-4361. https://academic.oup.com/eurheartj/article/43/41/4229/6673995.
- Zamorano JL, Lancellotti P, Muñoz DR, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). European Heart Journal. September 2016;37(36):2768-2801. https://academic.oup.com/eurheartj/article/37/36/2768/2197413.
- Anker MS, Ebner N, Hildebrandt B, et al. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: results of a prospective cardiovascular long-term study. European Journal of Heart Failure. December 2016;18(12):1524-1534. https://onlinelibrary.wiley.com/doi/10.1002/ejhf.670.
- Anker MS, Frey MK, Goliasch G, et al. Increased resting heart rate and prognosis in treatment‐naïve unselected cancer patients: results from a prospective observational study. European Journal of Heart Failure. March 2020;22(7):1230-1238. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7540544/.
- Mulrooney DA, Soliman EZ, Ehrhardt MJ, et al. Electrocardiographic abnormalities and mortality in aging survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. American Heart Journal. July 2017;189:19-27. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5477639/.