The use of artificial intelligence (AI) and deep learning reconstruction techniques is being more widely used in radiology as more clinical applications are developed. Specifically in magnetic resonance imaging (MRI), these techniques are advancing the capabilities of cardiac MRI (CMR) to visualize a variety of different cardiac conditions and provide insights into underlying disease and cardiac dysfunction. CMR is frequently used because it provides clinicians with accurate anatomic information along with advanced soft tissue contrast. CMR is considered the reference standard for quantification of the cardiac chamber size and systolic function of the heart1].
In a recent webinar, hosted by Society of Cardiovascular Magnetic Resonance (SCMR), sponsored by GE Healthcare, global experts in cardiology discussed clinical applications for CMR and their experiences using some of the newest AI technologies such as deep learning reconstruction in clinical cardiac MR practice. Alexander Hirsch, MD, PhD, Assistant Professor and Principal Investigator of Cardiovascular MRI Imaging at Erasmus Medical Center in Rotterdam, Netherlands presented a number of cases to illustrate effective tissue characterization techniques using AI-enhanced image reconstruction including late gadolinium enhancement (LGE), T1 mapping and T2 mapping. Shreyas Vasanawala, MD, PhD, New Division Chief, Associate Chair, and Radiologist-in-Chief for Pediatric Radiology of Stanford University in California highlighted how deep learning reconstruction can improve the pediatric cardiac experience with rapid and automated approaches to scanning this population.
Improving myocardial tissue characterization with deep learning reconstruction
Myocardial fibrosis is a commonly seen result of many cardiomyopathies and its impact on the heart is a major independent predictor of adverse cardiac outcomes2]. It is critical for these patients that their clinicians can accurately image and characterize myocardial tissue, which offers them insight into disease etiologies and the extent and stage of patients’ diseases.
Sophisticated CMR techniques are now in clinical practice that can provide myocardial tissue characterization beyond just confirming the presence of myocardial fibrosis[3]. These myocardial tissue characterization techniques include LGE, T1 mapping, and T2 mapping, which provide insight into the pathophysiological changes of the myocardium. These statistical parametric mapping techniques are image analysis tools that assesses the significance of blood flow changes in the myocardium.
Until now, the use of these techniques has been hindered by long acquisition times, inefficient protocol standardization, relatively low spatial resolution and high costs[4].
However, new software solutions using AI-enhanced reconstruction algorithms have accelerated the imaging acquisition times for statistical parametric mapping of the T1, T2, and also extracellular and cellular volume mapping and improved image quality achievable using LGE. Originally developed to image myocardial scarring, gadolinium contrast is often used to image tissue in patients with acute myocardial infarction. LGE is a useful tool for scar detection, based on differences in the volume of distribution of gadolinium over time. The increased amount of gadolinium can be demonstrated by T1-weighted imaging, in the time period of ten to thirty minutes after the contrast is administered.
Using GE Healthcare’s AIRTM Recon DL for AI-enhanced image reconstruction based on deep learning neural networks, Dr. Hirsch makes full use of patients’ complete raw imaging data sets for maximum image quality. The introduction of AI-enhanced image reconstruction improves the signal-to-noise (SNR) ratio and offers ringing suppression that preserves fine image details, helping address two common pain points for radiologists and technologists—image noise and ringing.
Dr. Hirsch presented some of the cases that were used in his recently published study in the European Journal of Radiology. The goal of the study was to assess the effects of using a deep learning-based reconstruction algorithm on LGE image quality and to evaluate its influence on scar quantification.
“In our study, we looked at 60 patients with known or suspected cardiomyopathy that underwent CMR using free-breathing LGE. Thirty of these patients had scars, ischemic heart disease, or a non-ischemic heart disease and fibrosis. We first looked at the sharpness of the images, specifically at the septum, where the blood goes into the myocardium. You can clearly see the improvements in the images that were reconstructed using the deep learning reconstruction. There is a remarkable increase in image quality at 25 percent noise reduction, and it further increases as you continue to increase noise reduction. What’s nice is that using the deep learning algorithm, you have a tunable noise reduction where you can set the noise reduction [to high, medium or low.] Using the deep learning reconstruction, you can see that the image quality was clearly improved.”
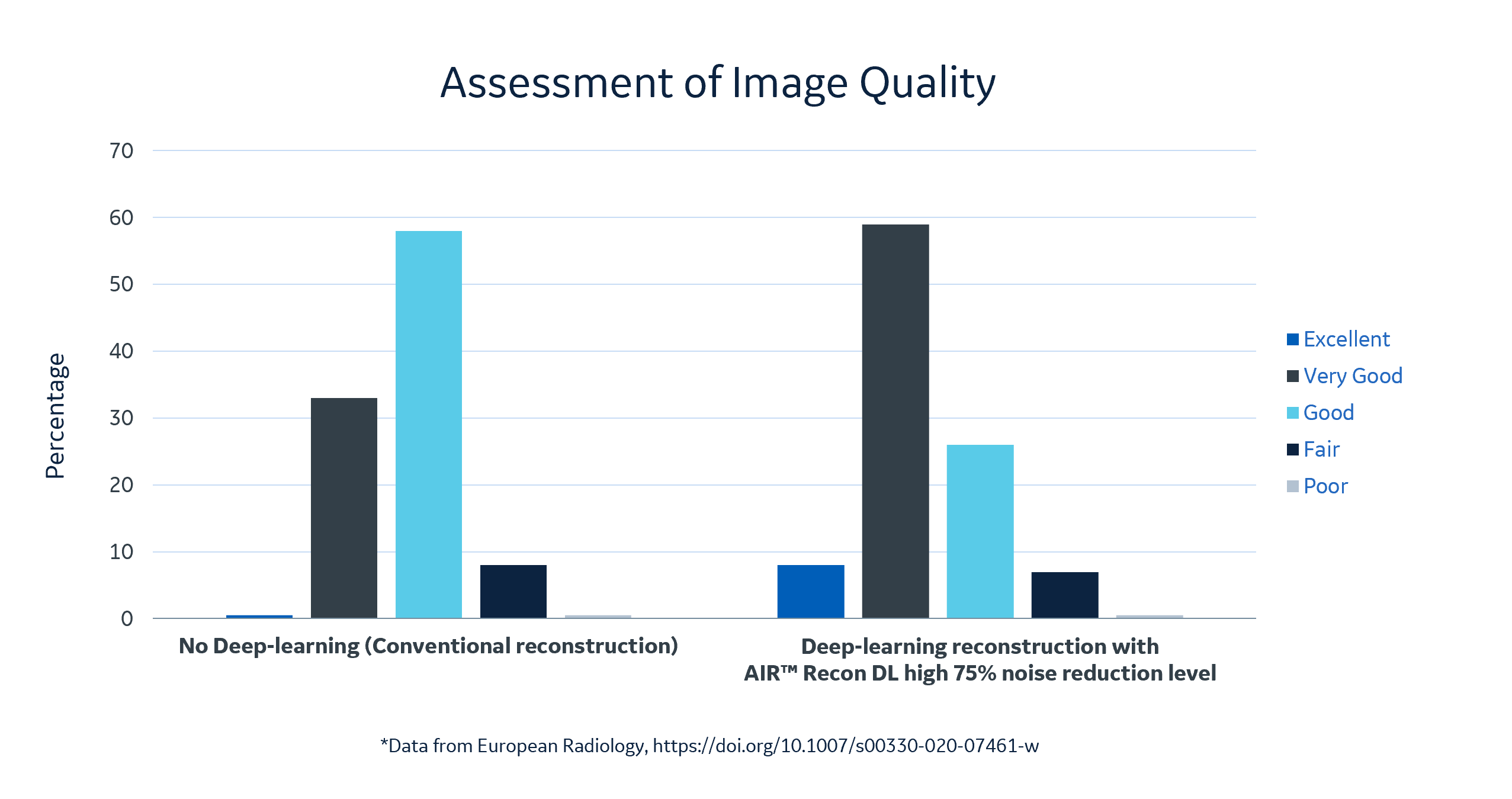
Dr. Hirsch explained that his research team assessed the image quality of the deep learning reconstruction images set at a 75 percent noise reduction level versus standard LGE images. The AI images earned high scores from the team, with eight percent excellent, 59 percent very good, and 26 percent good versus the non-deep learning enhanced reconstruction images with less than 1 percent excellent, 33 percent very good, 58 percent good. In 30 patients for ischemic heart disease scar size was also quantified on the LGE scans. The research team concluded that LGE image quality improved significantly using a deep learning-based reconstruction algorithm[5].
Dr. Hirsch concluded that deep learning image reconstruction could also be used for patients’ prognosis for diseases such as cardiac amyloidosis, and it positively impacts patient scan time as well.
“The deep learning reconstruction improves image quality by increasing image sharpness, and reducing noise and Gibb’s ringing, but what’s also possible is that you reduce scan time for your patients.”
Advantages of AI in Pediatric Cardiac MRI
According to Dr. Shreyas Vasanawala, there is a strong need for shortened scan times and efficient protocols in cardiac MRI, especially for the pediatric patients. They have limited ability to cooperate in the exam, for example holding still or holding their breath while holding still. For these reasons, pediatric cardiac MRI exams are often performed while patients are sedated with anesthesia.
The first of two rapid and automated pediatric MRI approaches discussed by Dr. Vasanawala was volumetric imaging. The goal of these congenital heart exams is threefold:
- defining segmental anatomy,
- quantifying flow in various vessels, and
- assessing the ventricular functions and size of the ventricles.
Dr. Vasanawala is able to achieve these goals in a single imaging sequence called the 4D flow scan.
“This volumetric approach, the 4D flow scan,” explained Dr. Vasanawala, “is performed with contrast enhancement and it is comprehensive. You’ll see that it provides both function and flow, as well as anatomy. And it’s great for technologists because the workflow is much simpler. You don’t have to have a detailed understanding of cardiac anatomy, and even better, you don’t have to have a detailed understanding of abnormal cardiac anatomy. Using deep learning image reconstruction, you get a volumetric scan that covers the whole chest, and after the fact, you can retroactively reformat images to see any plane you want.”
Dr. Vasanawala highlighted a single sequence acquisition that provided the same information as obtained by doing numerous conventional, lengthy scans. Looking at a short axis, two chamber and four chamber views, the AI automatically detects the landmarks of the heart and then performs the reformatting into these standard chamber views. He then illustrated the automation in vessel segmentation, which is automatically calculated to determine the flow in those vessels.
He pointed out that he can also segment out the ventricles and determine their cardiac output and ejection fractions, even in a case where the patient’s heart rate was exceptionally high, at 150 beats per minute, with very good image quality. He went on to explain the patient’s detailed cardiac anatomical findings and calculations as well.
“So if we look at this case,” Dr. Vasanawala concluded, “from this single sequence, we’ve obtained the following information. We know there’s a D-transposition of the arteries. We know it’s a bicuspid pulmonic valve, we saw the patient ductus arteriosus and quantified the flow through it. We also saw that inter-atrial communication, and also quantified the flow through it. We got a great look at the rich aortic arch and the anomalous left subclavian, and also quantified the shunt ratio as 4.3, and gave ejection fractions and the ventricular volume. So very nice quantitative exam from a single sequence that just covered the whole chest and did not require dedicated technologist training in cardiac MRI.”
Another approach Dr. Vasanawala discussed is conventional 2D imaging using AI for scanning and automated processing, and using a method that is free breathing, and therefore less taxing on the patient. The imaging approach focuses on automatically getting into the short axis plane, quantifying ventricular volumes and ejection fractions, as well as getting into the four-chamber view. Using a deep learning technique, the system automatically moves from an axial localizer into a pseudo two-chamber view and then from there into a four-chamber view, followed by a short-axis view.
Typically, Dr. Vasanawala explained, these images are obtained requiring multiple breath holds by the patient. Not only are they uncomfortable for the patient, they can lead to inaccurate quantification of ejection fractions. He has been working with GE Healthcare to develop a new, deep learning approach that allows for greatly accelerated acquisition*, and at the same time allows for respiratory triggering of the slices of those acquisitions, so there is no need for the patient to hold their breath.
Dr. Vasanawala and his team compiled a prospective study to review this approach.
“[On a] particular case, this was accelerated 12-fold, and what you see is if you just perform a simple zero-filled reconstruction, it’s highly accelerated and you have a lot of artifacts. Using a fully sampled acquisition, the images are high quality, but you can, even with a 12-fold acceleration, use more advanced reconstruction techniques such as compressed sensing or deep learning reconstruction, and you can recover the images quite nicely.”
When comparing the two advanced imaging techniques, Dr. Vasanawala highlighted the superior performance of the deep learning approach.
“What really matters to us at the end of the day is our cardiac output and ejection fraction measurements,” Dr. Vasanawala explained. “In an example of compressed sensing reconstruction and deep learning reconstruction, you can see that the tightness of the agreement, in terms of the left ventricular ejection fraction, versus a fully sampled scan is much tighter with the deep learning approach.”
Deep learning’s compelling data and future potential in CMR applications
Advances in MRI technology as well as AI-based innovations in image reconstruction have enabled compelling results thus far. As these solutions become more widely used across different CMR applications, they can provide clinicians with new insights into cardiac disease and treatments.
The clinical cases presented by Drs. Hirsch and Vasanawala illustrate the powerful improvements in image quality and in spatial resolution that were made possible using deep learning image reconstruction techniques. These innovations not only allow for improved image quality, but also ease the training burden on technologists with standardized image protocols, support a better patient experience with shortened exam times, and automate many of the calculations radiologists need to diagnose cardiac disease.
As innovation in AI for CMR applications continues, it has the potential to help clinicians detect abnormalities that are much more subtle than can be seen with the human eye, and continue to support radiologists’ role as an essential diagnostician in cardiology.
RELATED CONTENT
To access the on-demand webinar, The Advantages of Deep Learning in Clinical Cardiac MR Practice, and to see the case studies presented by Drs Hirsch and Vasanawala, click here.
For more information on GE Healthcare’s AIRTM Recon DL, click here.
For more information on GE Healthcare’s Cardiac MR solutions, click here.
DISCLAIMER
Not all products or features are available in all geographies. Check with your local GE Healthcare representative for availability in your country.
*Technology in development that represents ongoing research and development efforts. These technologies are not products and may never become products. Not for sale. Not cleared or approved by the US FDA or any other global regulator for commercial availability.
REFERENCES
[1] Lee SE, Nguyen C, Xie Y, et al. Recent Advances in Cardiac Magnetic Resonance Imaging. Korean Circ J. 2019;49(2):146-159. doi:10.4070/kcj.2018.0246, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351278/
[2] Pattanayak P, Bleumke DA. Tissue characterization of the myocardium: state of the art characterization by magnetic resonance and computed tomography imaging. Radiol Clin North Am. 2015;53(2):413-423. doi:10.1016/j.rcl.2014.11.005, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4348002/
[3] Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) [published correction appears in J Cardiovasc Magn Reson. 2018 Feb 7;20(1):9]. J Cardiovasc Magn Reson. 2017;19(1):75. Published 2017 Oct 9. doi:10.1186/s12968-017-0389-8, https://pubmed.ncbi.nlm.nih.gov/28992817/
[4] Lee SE, Nguyen C, Xie Y, et al. Recent Advances in Cardiac Magnetic Resonance Imaging. Korean Circ J. 2019;49(2):146-159. doi:10.4070/kcj.2018.0246, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6351278/
[5] van der Velde N, Hassing HC, Bakker BJ, et al. Improvement of late gadolinium enhancement image quality using a deep learning-based reconstruction algorithm and its influence on myocardial scar quantification [published online ahead of print, 2020 Nov 21]. Eur Radiol. 2020;10.1007/s00330-020-07461-w. doi:10.1007/s00330-020-07461-w, https://pubmed.ncbi.nlm.nih.gov/33219845/