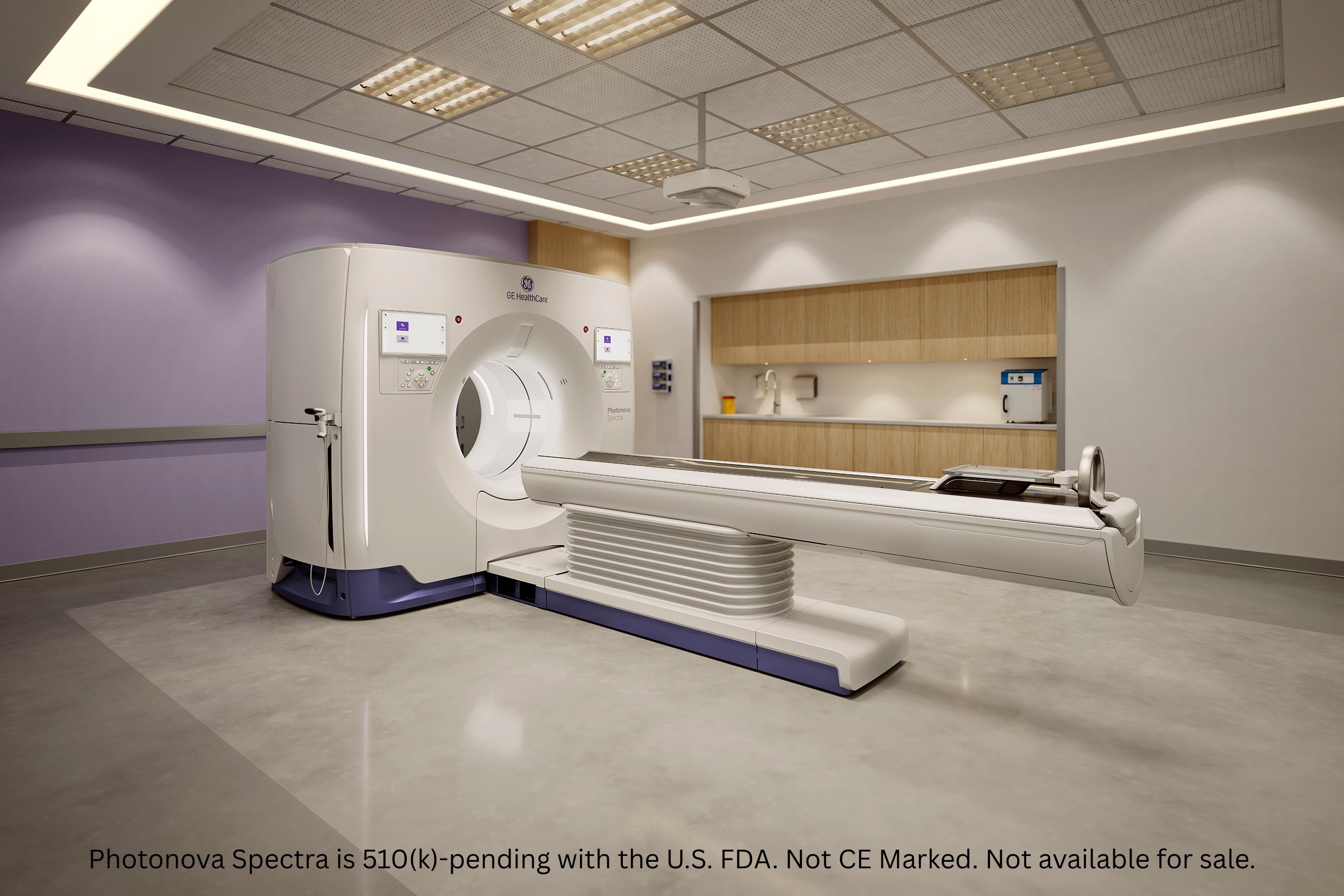
* Photonova Spectra is 510(k)-pending with the U.S. FDA. Not CE Marked. Not available for sale.
Three years after spinning off from General Electric, GE HealthCare is entering what company executives call a “defining chapter”—a period marked by bold, purposeful innovation aimed at reshaping patient care and hospital efficiency. At the center of this push is a technology that aims to redefine diagnostic imaging: photon counting CT.
Why photon counting matters
For decades, CT imaging has relied on detectors that convert X-rays into light before measuring them. Photon counting CT flips that paradigm. Instead of converting photons, it counts each individual X-ray photon and measures its energy, enabling the potential for higher spectral and spatial resolution and improved tissue characterization.
“Photon counting CT is a fundamentally different approach to imaging. It can be thought of as particle physics in action,” shares Giuseppe Toia, MD, Assistant Professor of Radiology, Associate Section Chief of Abdominal Imaging and Intervention and Program Director of Abdominal Imaging and Intervention Fellowship with the Department of Radiology at the University of Wisconsin School of Medicine and Public Health. “The goal of using photon counting CT is to help differentiate materials and reveal diagnostic details, which is of interest to radiologists for informed clinical decision-making and streamlined workflows.”
The promise of this technology is profound, seeking to enable fast acquisition speeds, precise visualization of anatomical structures and enhanced material separation. For clinicians, it aims to provide and empower them with sharp images, rich data and confident diagnoses.
Photonova™ Spectra:[i] See more, know more, achieve more
GE HealthCare’ s 510(k) pending, next generation photon counting CT solution, Photonova Spectra,[i] is designed to realize the technology’s potential. Built on the company’s proprietary Deep Silicon detector technology, Photonova Spectra is designed to deliver ultra-high-definition (UHD) imaging, wide coverage, and advanced spectral and spatial capabilities—all in one scan.
Unlike conventional systems, Photonova Spectra leverages eight energy bins to capture precise spectral information, aiming to support advanced lesion characterization and treatment monitoring with CT. It’s designed for impact across specialties—from neurology and oncology to musculoskeletal and cardiac imaging.
“Clinicians need clear answers as patient volumes rise[ii] and cases grow more complex,”[iii] says Chad Rowland, General Manager, Photon Counting CT and Premium CT, GE HealthCare. “Photonova Spectra was engineered for that reality—leveraging Deep Silicon detectors to deliver ultra-high-definition imaging with wide coverage and fast acquisition. It is designed to deliver precise visualization of subtle tissue variations, small lesions and vascular structures in one scan, with the aim of providing clinicians the confidence to diagnose and plan treatment quickly.”
A Deep Silicon detector chicklet in production at GE HealthCare’s Waukesha, WI CT innovation hub.
The Deep Silicon difference
GE HealthCare is proud to introduce a new photon counting detector technology built with a key differentiator: silicon. Why silicon? For impressive accuracy, sustainability, and scalability.
“Silicon is abundant, derived from sand, and one of the most researched materials,” explains Rowland. “Its purity and structural consistency enables the precise measurement of photon energy and delivers high levels of energy resolution – critical for advanced image reconstruction. This wasn’t just a material choice; it was a strategic decision to build a new CT detector technology that can evolve and meet the growing demands of precision medicine.”
Each detector module contains 4,800 sensor elements, assembled with micron-level precision and millions of gold wire bonds. Once assembled, these detectors are placed in the CT’s gantry, which is capable of spinning at forces exceeding 50 Gs—moving the mass of a Mini Cooper around a patient’s body up to four times per second—while maintaining tolerances within the thickness of a sheet of paper. Achieving that balance is an engineering feat years in the making.
Managing the data deluge
GE HealthCare’s Photonova Spectra is designed to maximize the vast amounts of data provided, harnessing up to 50 times more data than conventional CT[iv] with the help of NVIDIA’s accelerated computing technology to enable advanced reconstruction techniques and precise outputs with the aim of supporting enhanced clinical decision-making and smooth workflows.
“Accelerated computing and AI are the essential engines driving the transformation of medical imaging today, moving us from passive data capture to active, intelligent clinical workflows,” notes Kimberly Powell, Vice President of Healthcare at NVIDIA. “This collaboration is helping reshape what is possible in diagnostic imaging and aiming to set a new standard for clinical efficiency and precision.”
The difference between seeing and knowing
The clinical potential of Photonova Spectra with Deep Silicon seeks to enable precise visualization of anatomical structures and enhanced material separation. Its UHD imaging and 8-bin spectral technology design automatically capture both spectral and spatial data in every scan—no special setup or multiple protocols required. This design aims to deliver clinicians the information they need in one exam, supporting confident decisions across specialties.
The system’s clinical design goals include:
-
Neurology: Excellent visualization of tiny structures like the inner ear and clear differentiation between brain grey and white matter at the same time.[v]
-
Oncology: Clear lesion characterization and precise quantification due to the system’s Deep Silicon detectors – helping clinicians make confident decisions for cancer detection. Its iodine mapping also aims to help clinicians distinguish oncological findings and support treatment monitoring.
-
Musculoskeletal imaging: Impressive visualization of small fractures and bone marrow edema, supporting detailed assessments for orthopedic care.
-
Thoracic imaging: Ultra-high-definition chest scans, capable of revealing fine details with exceptional clarity.
-
Cardiology: One second acquisitions support rapid cardiac scans and full chest imaging to enable in-stent lumen assessment, plaque characterization and myocardial assessment.
The bigger picture
GE HealthCare’s investment in photon counting CT is part of a broader strategy: $3 billion invested in research and development (R&D) since 2022, fueling more than 20 technologies across system, AI and cloud solutions. The company’s objective is clear — deliver smarter, faster, and more connected care.
For more information on GE HealthCare’s new Photonova Spectra photon counting CT with Deep Silicon detectors, please visit gehealthcare.com or stop by the company’s booth (7334) at the Radiological Society of North America’s (RSNA) 2025 Annual Meeting in Chicago.
[i] Photonova Spectra is 510(k)-pending with the U.S. FDA. Not CE Marked. Not available for sale in the United States, Europe, Canada, or any other region.
[ii] Kaufman Hall. State of Hospital Volumes. Kaufman Hall, https://www.kaufmanhall.com/insights/infographic/state-hospital-volumes. Accessed October 6, 2025.
[iii] Sittig, Dean F., and Hardeep Singh. A New Socio-technical Model for Studying Health Information Technology in Complex Adaptive Healthcare Systems. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 1: Assessment). Rockville (MD): Agency for Healthcare Research and Quality (US); August 2008. https://www.ncbi.nlm.nih.gov/books/NBK338593/. Accessed October 6, 2025.
[iv] When compared to Revolution Apex Elite.
[v] Enhanced Boundary for PCCT is 510(k)-pending with the U.S. FDA. Not CE Marked. Not available for sale in the United States, Europe, Canada, or any other region.