The global rate of breast cancer has grown substantially over the past decade. Female breast cancer has now surpassed lung cancer as the most diagnosed cancer in the world, with more than 2 million new cases identified each year.¹ In tandem, mortality rates for this type of cancer are also on the rise, with a significantly larger proportion of deaths occurring in women who live in developing countries.1 Unfortunately, the burden of this disease is only expected to grow over the next two decades.
Given such dire predictions, the importance of early detection of breast tumors cannot be overstated. Detecting tumors early—when they are still small and have not yet metastasized to other parts of the body—is key to preventing breast cancer deaths. Early detection also increases the likelihood that the cancer can be treated more successfully, less invasively, and at a reduced cost.
Today, clinicians all over the world rely on mammography to help them screen for breast cancer. This X- ray-based technology is the gold standard for detecting the hallmark microcalcifications and masses that denote potential breast cancer. That is why the American Cancer Society, as well as other clinical bodies, recommend that all women between the ages of 45 and 54 get a mammogram each year.2 Similarly, the European Union recommends women between the ages of 50 and 69 are regularly screened every two years.3
Yet, while mammography remains the gold standard for identifying masses that require further evaluation through secondary screening or biopsy, it is not infallible. Many women will eventually find themselves diagnosed with breast cancer despite receiving one or more negative screening results. This is particularly common in women who have dense breast tissue. In fact, studies show that traditional mammography misses one-third of cancers in patients with this characteristic.4
The Need for Different Tests for Dense Breasts
No two breasts are the same. Each one is made up of a unique mixture of fatty, fibrous, and glandular tissue. When there is more fibrous and glandular than fatty tissue in the breast, it is considered to be dense. Dense breasts are completely normal. In fact, more than 40 percent of women in the United States have dense breasts.5
That percentage can go up to 70 percent for women living in other parts of the world.6 But dense tissue can present a significant challenge for reading mammography images. Dense tissue appears white on the image. As masses or lumps are similarly hued, they are often hidden from view. That’s why some radiologists say looking for potential masses in a mammogram of a dense breast is akin to “looking for a snowball in a snowstorm.”7
71% of all breast cancers occur in patients with dense breasts.8
Given that this type of tissue is a significant risk factor for the development of breast cancer—71 percent of all breast cancers occur in patients with dense breasts8—successful screening presents a tremendous clinical challenge to healthcare. Many cancers may be missed in a routine mammography simply because potential masses may be camouflaged by the dense tissue surrounding them.9 That is why there is a current push, both in the United States and beyond, to raise awareness of the issues of breast density in mammography screening. Thirty-eight states now have laws in place mandating that clinicians notify patients if they have dense breasts and the resulting increased risk for certain types of cancer. Thirteen of those states now also require that insurance cover costs for supplemental screening.
To help ensure women can benefit from early detection, clinicians need additional tools to reliably screen for cancer in this unique population of patients.
To help ensure women can benefit from early detection, clinicians need additional tools to reliably screen for cancer in this unique population of patients. The GE Invenia™ ABUS 2.0, the first Food and Drug Administration (FDA) approved ultrasound device for supplemental screening for women with dense breasts, can provide radiologists with a rapid, reliable screening tool to detect many of the cancers that mammography may miss in this patient population.
Separating Image Acquisition from Diagnosis to Speed Results
Today, many patients with dense breasts will be referred for a secondary screening with traditional ultrasound. This handheld screening requires a trained radiologist or sonographer to perform both the scan and diagnosis in real time. As such, patients with dense breasts often face a delay in scheduling this supplemental screening due to appointment bottlenecks—and, because diagnosis is as much of an art as it is a science, the results are only as good as the operator performing the scan.
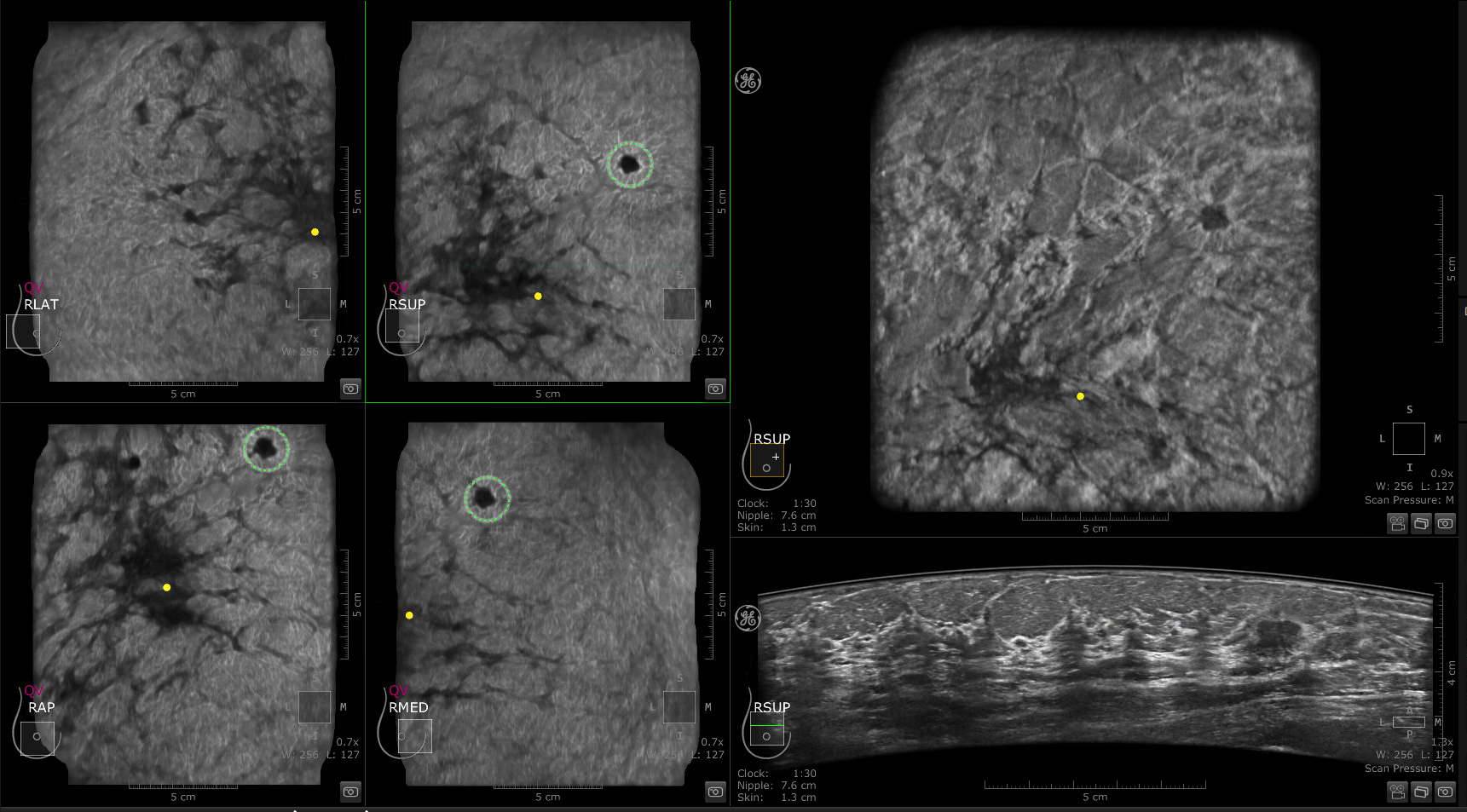
Using the GE Invenia ABUS 2.0, gives radiologists the ability to separate the acquisition of ultrasound images and the reading of the results. The patented Reverse Curve™ transducer moves automatically, generating a comprehensive, three-dimensional image of each breast. These full volume images are then transferred to a workstation or picture archiving and communications system (PACS) where they can be reviewed by the physician. This novel, standardized platform offers radiologists superior speed in the generation of images.
This new standardized platform can reduce the average reading time for a complete ABUS study to 3 minutes.
According to Dr. Athina Vourtsis, Diagnostic Mammography Athens, Greece, the average reading time for physicians for a complete ABUS study is approximately 3 minutes.10 The ABUS also boasts improved clinical accuracy compared to traditional ultrasound, as the coronal plane provides superiority to HHUS, especially in the detection of architectural distortions – a finding highly suspicious for malignancy.
In 2016, the Fort Jesse Imaging Center and Gale Keeran Center for Women in Central Illinois installed the GE Invenia ABUS 2.0 solution to round out its supplemental screening program. Over the four-year study period, the radiology groups at these two imaging centers provided more than 40,000 mammograms. Approximately 38 percent of those women were found to have dense breast tissue.11
New screening resulted in greater cancer detection in patients with dense breasts, even identifying lesions as small as 0.1 centimeters.11
When the radiology group compared supplemental screening results between mammography and the ABUS solution, they found ABUS resulted in greater cancer detection in patients with dense breasts, even identifying lesions as small as 0.1 centimeters. The majority of the cancers detected by ABUS were invasive varieties but, since they were identified while still at grades 1 and 2, had a higher probability of successful treatment outcomes.11
The AI Advantage: Faster Processing, Higher Detection Sensitivity, Improved Clinical Accuracy
The assistance of artificial intelligence (AI) technology provides enhanced diagnostic capabilities with the assistance of artificial intelligence (AI) technology. The GE Invenia ABUS 2.0 has an open platform that leverages third-party AI tools such QVCAD™* and Koios DS™* Breast. The lesion detector powered by QView Medical, has been demonstrated to provide up to 93 percent sensitivity for lesion detection, as well as a 33 percent reduction in reading time.12,13
A lesion detector powered by QView Medical, demonstrated up to 93 percent sensitivity for lesion detection, as well as a 33 percent reduction in reading time.12,13
Jonathan T. Sims, MD, MBA, Radiologist at Oregon Imaging Centers shares: “CAD is a great tool; it acts like a second read for the ABUS exams. CAD has improved our workflow by reducing the reading time and further need for traditional handheld Breast Ultrasound exams.” Jon E. Ekstrom, MD, Radiologist at Oregon Imaging Center, also adds: “The implementation of CAD on the ABUS Viewer streamlines workflow, helping us prioritize areas to focus on. Based on my experience, I believe CAD could help reduce ABUS reading time by 50 percent.”
“CAD has improved our workflow by reducing the reading time and further need for traditional handheld Breast Ultrasound exams.”
-- Dr. Jonathan Sims, Oregon Imaging Centers
“The implementation of CAD on the ABUS Viewer streamlines workflow, helping us prioritize areas to focus on. Based on my experience, I believe CAD could help reduce ABUS reading time by 50%.”
-- Dr. Jon Ekstrom, Oregon Imaging Centers
IDC detected with QVCAD and ABUS
A decision support tool powered by Koios Medical, can improve diagnosis and clinical decision making, decreasing the number of biopsies performed on benign masses by up to 31 percent.14 Leveraging this technology provides clinicians a proven, operator independent screening tool, offering fast, reproducible results and high clinical impact.
AI decision support powered by Koios Medical decreased the number of biopsies performed on benign masses by up to 31 percent.14
This Invenia ABUS Scanner, powered by Intel® Core™ processors, provides clinicians with high-resolution, full volume images to aid in detection and diagnosis. These processors support the high-speed data transfer through direct memory access (DMA), to provide accurate and reliable image reconstruction.
GE Healthcare and Intel: Partnering to Deliver Extraordinary Image Quality
The Intel CPU ensures no frame is dropped—and that the system can rapidly achieve the required frame rates for image acquisition and rendering. In addition, Intel® processors also help the GE Invenia ABUS 2.0 platform enhance images as needed. This kind of computing performance helps ensure uncompromised image quality, as well as fast image acquisition and interpretation, in a compact, mobile solution. No trade-off between speed and accuracy is required.
This kind of computing performance helps ensure uncompromised image quality, as well as fast image acquisition and interpretation, in a compact, mobile solution. No trade-off between speed and accuracy is required.
The reading radiologist will be able to easily view the right details in the breast image in a streamlined fashion so he or she can make a quick and accurate diagnosis. In addition, Intel technology helps the GE Invenia ABUS 2.0 platform strike the right balance between price and computing performance. Intel has long provided industry leading performance with cost-effective, standards-based platforms.
By relying on Intel CPUs, GE Healthcare has built a powerful, standards-based, integrated solution to meet the needs of today’s radiologists as they work to offer earlier detection to patients with dense breast tissue.
The Global Impact of Improving Identification of T1 Stage Breast Cancers
The ability to identify a greater number of T1 stage breast cancers can help to reduce the burden of breast cancer around the globe, providing direct benefits to clinicians, patients, and society at large.15 The use of GE Invenia ABUS 2.0, with its enhanced detection capabilities powered by highspeed Intel processors, offers radiologists superior clinical confidence as they review screening images of patients with dense breast tissue.
With enhanced imaging tools, those clinicians will be more likely to identify smaller, node negative cancers in these patients, long before metastasis, increasing the likelihood that those tumors will respond to less invasive, less expensive, and more effective treatments. Finally, the addition of this standardized, automated breast ultrasound solution increases the value of breast cancer screening across the board, helping to reduce the burden of breast cancer morbidity and mortality in the United States and abroad.
Learn more about Invenia ABUS
https://www.gehealthcare.com/products/ultrasound/abus-breast-imaging/invenia-abus
https://www.youtube.com/watch?v=BzwcStFxTzA
Hear from the Experts
https://www.abusclub.net/us/generalnews
REFERENCES
1 Sung Hyuna, et al., “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.” CA: A Cancer Journal for Clinicians, 71, No. 3 (2021): 209–249.
2 American Cancer Society. “American Cancer Society Recommendations for the Early Detection of Breast Cancer.” https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society- recommendations-for-the-early-detection-of-breast-cancer.html
3 European Commission “European Breast Cancer Guidelines: Screening Ages and Frequencies.”https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines/screening-ages-and-frequencies
4 Kolb, Thomas M., et al, “Comparison of the Performance of Screening Mammography, Physical Examination, and Breast US and Evaluation of Factors that Influence Them: An Analysis of 27,825 Patient Evaluations.” Radiology 225, No. 1 (2002): 165–75.
5 Pisano, Etta D., et al., “Diagnostic Performance of Digital versus Film Mammography for Breast-Cancer Screening.” The New England Journal of Medicine 353 (October 27, 2005): 1773–1783.
6 Jakes, RW, et al., “Mammographic Parenchymal Patterns and Risk of Breast Cancer at and after a Prevalence Screen in Singaporean Women.” International Journal of Epidemiology 29, No. 1 (April 2000): 11–19.
7 GE Healthcare. (2018). “All breasts are not the same. https://www.gehealthcare.com/-/jssmedia/files/g/2019/01/28/globalinveniaabus20densityquickfactssheetdecember2018.pdf?rev=-1
8 Arora, Nimmi, et al., “Impact of Breast Density on the Presenting Features of Malignancy.” Annals of Surgical Oncology 17 (2010): 211–218.
9 Mandelson, M.T., et al., “Breast Density as a Predictor of Mammographic Detection: Comparison of Interval- and Screen-Detected Cancers.” Journal of National Cancer Institute 92, No. 13 (July 5, 2000): 1081–1087.
10 Vourtsis, et al. “The performance of 3D ABUS versus HHUS in the Visualisation and BI-RADS characterization of Breast Lesions in a large cohort of 1,886 women”. European Radiology DOI 10.1007/s00330-017-5011-9
11 GE Healthcare. (2021). Continued Growth and Outcomes with Invenia ABUS (Automated Breast Ultrasound) at Fort Jesse’s Imaging Center and Gale Keeran Center for Women.
12 Yang, Shanling, et al., “Performance and Reading Time of Automated Breast US with or without Computer-aided Detection.” Radiology 292, No. 3 (June 18, 2019): https://doi.org/10.1148/radiol.2019181816
13 Jiang, Yulei, et al. “Interpretation Time Using a Concurrent-Read Computer-Aided Detection System for Automated Breast Ultrasound in Breast Cancer Screening of Women with Dense Breast Tissue.” American Journal of Roentgenology 211, No. 2 (August 2018): 452–461. https://www.ajronline.org/doi/10.2214/AJR.18.19516
14 Mango, Victoria L., et al. “Should We Ignore, Follow, or Biopsy? Impact of Artificial Intelligence Decision Support on Breast Ultrasound Lesion Assessment.” American Journal of Roentgenology 214, No. 6 (June 2020): 1145–1452. https://www.ajronline.org/doi/full/10.2214/AJR.19.21872
15 Blumen, et al. Comparison of treatment costs for breast cancer by tumor stage, and type of service.
*Not all products are available in all regions or countries, consult your GE Healthcare representative for availability in your region.